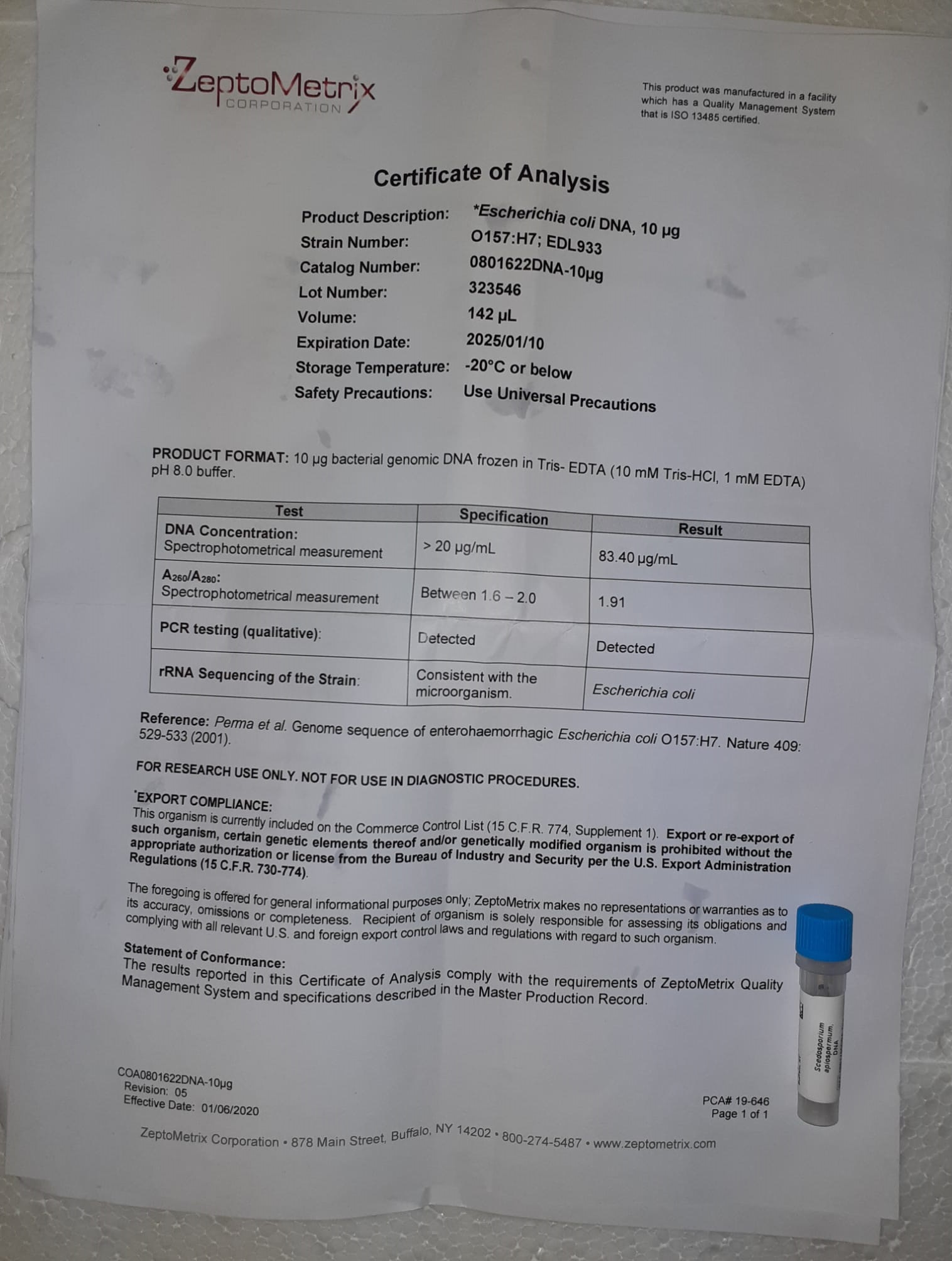
The diagnostic impact of UK regional variations in age-specific prostate-specific antigen guidelines
The perfect prostate most cancers diagnostic pathway would maximise detection of clinically-significant prostate most cancers (csPCa) whereas avoiding pointless biopsies and different investigations. The introduction of pre-biopsy MRI has completed a lot to help this aim. Nevertheless, referrals into the image-based diagnostic pathway nonetheless depends upon prostate-specific antigen (PSA) testing carried out in major care and interpreted utilizing referral pointers.
Within the UK, the Nationwide Institute for Well being and Care Excellence (NICE) solely supplies steering on PSA thresholds for males aged 50-69 years (PSA ≥3.zero ng/mL) [1]. For different age teams, PSA thresholds are set by regional most cancers networks with none unified consensus. Right here we explored if totally different regional pointers impacted csPCa detection in trendy image-based pathways.
HLA-DR-Optimistic NK Cells Increase in Response to Mycobacterium Tuberculosis Antigens and Mediate Mycobacteria-Induced T Cell Activation
NK cells play an vital function within the management of tuberculosis an infection: they don’t seem to be solely in a position to kill the contaminated cells, but in addition management the exercise of macrophages and improvement of the adaptive immune response. Nonetheless, there’s little data on the function of particular NK cell subsets on this community. On this research, we targeted on the mycobacteria-driven responses of the NK cells expressing HLA-DR – a kind of MHC class II. Now we have revealed that this subset is elevated within the peripheral blood of sufferers with major recognized tuberculosis, and expands in response to in vitro stimulation with ultrasonically destroyed Mycobacterium tuberculosis cells (sonicate).
The expanded HLA-DR+ NK cells had much less differentiated phenotype, greater proliferative exercise and elevated expression of NKp30 and NKp46 receptors. HLA-DR+CD56dim NK cells confirmed greater IFNγ manufacturing and degranulation degree than the respective HLA-DR– NK cells in response to each 24 h and seven day stimulation with sonicate, whereas HLA-DR+CD56vivid NK cells principally demonstarted related excessive responsiveness to the identical stimulating circumstances as their HLA-DR–CD56vivid counterparts. After preliminary incubation with destroyed mycobacteria, cytokine-activated HLA-DR-expressing NK cells have been in a position to mediate mycobacteria-induced and HLA-DR-dependent cytokine manufacturing in autologous CD4+ T cells. Thus, functionally lively HLA-DR+ cells appear to be one of many NK cell subsets offering an vital hyperlink to the adaptive immunity.
Design of the SARS-CoV-2 RBD vaccine antigen improves neutralizing antibody response
The receptor binding area (RBD) of the SARS-CoV-2 spike protein is the first goal of neutralizing antibodies and is a part of virtually all vaccine candidates. Right here, RBD immunogens have been created with stabilizing amino acid modifications that enhance the neutralizing antibody response, in addition to traits for manufacturing, storage, and distribution. A computational design and in vitro screening platform recognized three improved immunogens, every with roughly 9 amino acid modifications relative to the native RBD sequence and 4 key modifications conserved between immunogens.
The modifications are adaptable to all vaccine platforms, are suitable with established modifications in SARS-CoV-2 vaccines, and are suitable with mutations in rising variants of concern. The immunogens elicit greater ranges of neutralizing antibodies than native RBD, focus the immune response to structured neutralizing epitopes, and have elevated manufacturing yields and thermostability. Incorporating these variant-independent amino acid modifications in next-generation vaccines might improve the neutralizing antibody response and result in pan-SARS-CoV-2 safety.

Modulating T Follicular Cells In Vivo Enhances Antigen-Particular Humoral Immunity
Era of high-affinity IgG is important for protection in opposition to infections and most cancers, which is the supposed consequence of many vaccines, however could cause autoimmune and inflammatory ailments when inappropriately directed in opposition to self. The interaction of T follicular helper (TFH) cells and T follicular regulatory (TFR) cells is important for the manufacturing of high-affinity IgG of a selected subclass. On this research, we sought to enhance Ag-specific IgG responses with two interventions supposed to transiently diminish TFR cell affect. First, grownup mice have been administered an antibiotic combination (ABX) for an prolonged interval to deplete the immunoregulatory intestinal microbiota.
This intriguingly elevated TFH cell and diminished TFR cell numbers. 2,4,6-Trinitrophenyl hapten conjugated to keyhole limpet hemocyanin immunization resulted in greater affinity 2,4,6-trinitrophenyl hapten-specific IgG1 in ABX mice in contrast with controls. In a mannequin of IgG-driven inflammatory nephritis, ABX mice had considerably worse nephritis accompanied by greater affinity Ag-specific IgG2b and enriched TFH cells in contrast with controls. Second, we sought to functionally manipulate TFH and TFR cells, which each specific the checkpoint inhibitory molecule, PD-1, by administration of anti-PD-1 throughout immunization. This intervention enhanced the affinity of Ag-specific IgG of the applicable subclass and elevated in TFH cells following 2,4,6-trinitrophenyl hapten conjugated to keyhole limpet hemocyanin immunization and nephritis induction. These outcomes recommend that altering TFH and TFR cell ratios throughout immunization is an interesting technique to qualitatively enhance Ag- and subclass-specific IgG responses.
Human delta like 1-expressing human mesenchymal stromal cells promote human T cell improvement and antigen-specific response in humanized NOD/SCID/IL-2R[Formula: see text] null (NSG) mice
Human delta-like 1 (hDlk1) is understood to have the ability to regulate cell destiny choices throughout hematopoiesis. Mesenchymal stromal cells (MSCs) are recognized to exhibit potent immunomodulatory roles in a wide range of ailments. Herein, we investigated in vivo features of hDlk1–hMSCs and hDlk1+hMSCs in T cell improvement and T cell response to viral an infection in humanized NOD/SCID/IL-2Rγnull (NSG) mice. Co-injection of hDlk1–hMSC with hCD34+ wire blood (CB) cells into the liver of NSG mice markedly suppressed the event of human T cells. In distinction, co-injection of hDlk1+hMSC with hCD34+ CB cells into the liver of NSG dramatically promoted the event of human T cells. Human T cells developed in humanized NSG mice symbolize markedly numerous, functionally lively, TCR V[Formula: see text] usages, and the restriction to human MHC molecules.
Upon problem with Epstein-Barr virus (EBV), EBV-specific hCD8+ T cells in humanized NSG mice have been successfully mounted with phenotypically activated T cells offered as hCD45+hCD3+hCD8+hCD45RO+hHLA-DR+ T cells, suggesting that antigen-specific T cell response was induced within the humanized NSG mice. Taken collectively, our knowledge recommend that the hDlk1-expressing MSCs can successfully promote the event of human T cells and immune response to exogenous antigen in humanized NSG mice. Thus, the humanized NSG mannequin may need potential benefits for the event of therapeutics focusing on infectious ailments sooner or later.